
Setra CEMS can help regulated manufacturing spaces comply with 21 CRF Part 11. Companies to which 21 CFR Part 11 applies, as well as related businesses, must guarantee both electronic records and signatures are accurate and reliable.Īs a US Federal regulation, 21 CFR Part 11 covers the following to guarantee accuracy and reliability of electronic records:
Part 11 file monitor software#
A software based continuous environmental monitoring solution provides:
Part 11 file monitor manual#
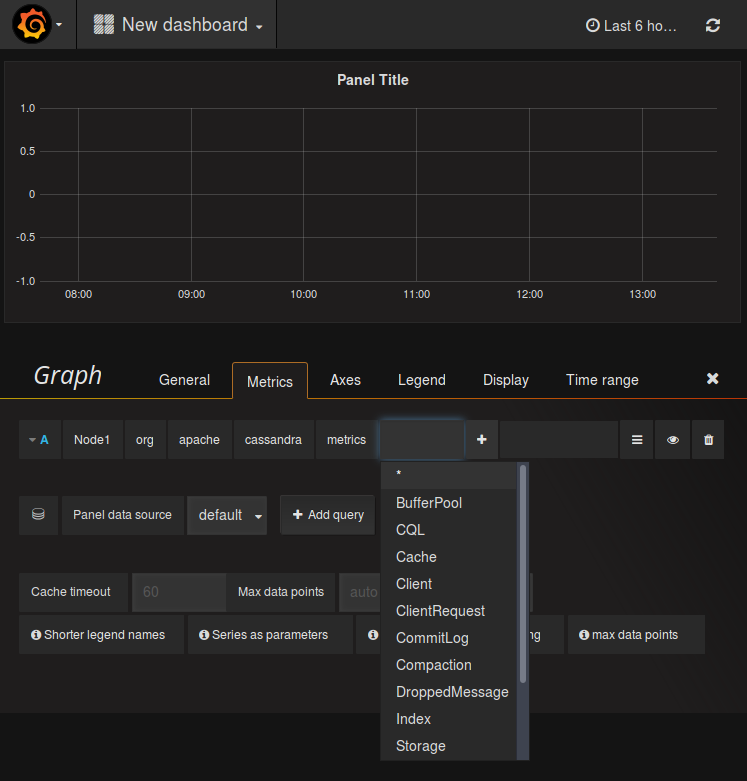
A software based continuous environmental monitoring solution eliminates many of the potential issues associated with manual monitoring. However, the trend in cleanroom monitoring is moving toward electronic, continuous monitoring solutions that put data integrity first.
As such, there is a higher risk of errors or inconsistences in the collected data. OverviewĮnvironmental data monitoring and recording is often a manual, human-centric process. When it comes to how data is handled in regulated cleanroom environments, the United States FDA issued 21 CFR Part 11 guidance that should be followed closely. Cleanrooms for medical device and pharmaceutical manufacturing are highly regulated spaces and there are numerous standards that need to be considered.


 0 kommentar(er)
0 kommentar(er)
